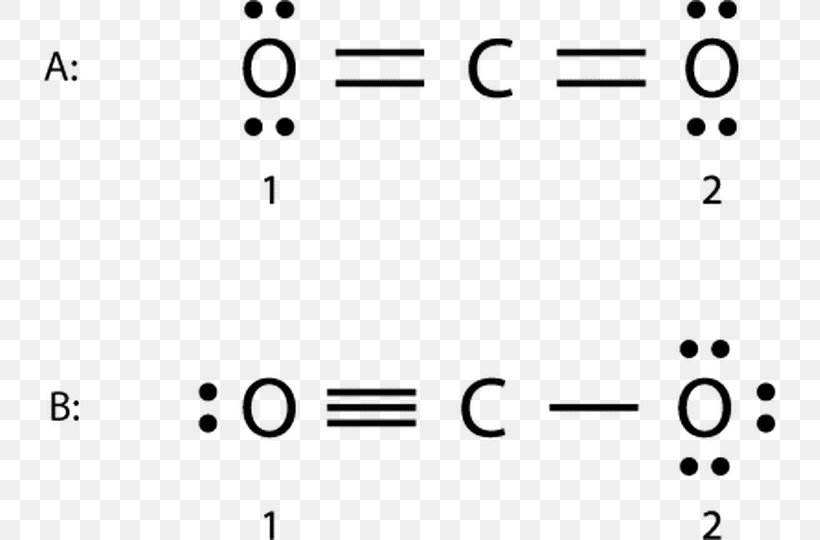
Co2 Dot Structure
A step-by-step explanation of how to draw the Lewis Dot Structure for Carbon dioxide (CO2 ).For the Carbon dioxide structure we use the periodic table to fi.

CO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram
The Lewis structure is a structure that shows the bonding between atoms as short lines (some books use pairs of dots), and non-bonding valence electrons as dots. 1.2.1 Lewis Structure of Diatomic Molecules. To learn about Lewis structures, we will start with the Lewis symbol. The Lewis symbol is the chemical symbol of an element with valence.

CO2 Lewis Structure ,Valence Electrons, Formal Charge ,Polar or
The N atom has the following Lewis electron dot diagram: It has three unpaired electrons, each of which can make a covalent bond by sharing electrons with an H atom. The electron dot diagram of NH 3 is as follows: Exercise 12.4.2 12.4. 2. Use a Lewis electron dot diagram to show the covalent bonding in PCl 3. Answer.

What is the Lewis Dot structure for CO2 (Carbon dioxide)?
Lewis Structure Finder. This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
CO2 Lewis Structure ,Valence Electrons, Formal Charge ,Polar or
The Lewis structure helps us identify the bond pairs and the lone pairs. Then, with the Lewis structure, we apply the valence-shell electron-pair repulsion (VSPER) theory to determine the molecular geometry and the electron-group geometry.. Carbon dioxide has two electron groups and no lone pairs. Carbon dioxide is therefore linear in.

Lewis Structure Carbon Dioxide Co2 Stock Vector (Royalty Free
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, and.

What is the Lewis Dot structure for CO2 (Carbon dioxide)?
For Lewis structure of CO2, you will now have two Oxygen atoms forming double bonds with a Carbon atom. As all the valence electrons of all the atoms are used, there are no lone pairs of electrons or non-bonding pairs of electrons in the molecule. To further understand the molecular geometry of CO2, let us quickly go through its hybridization.
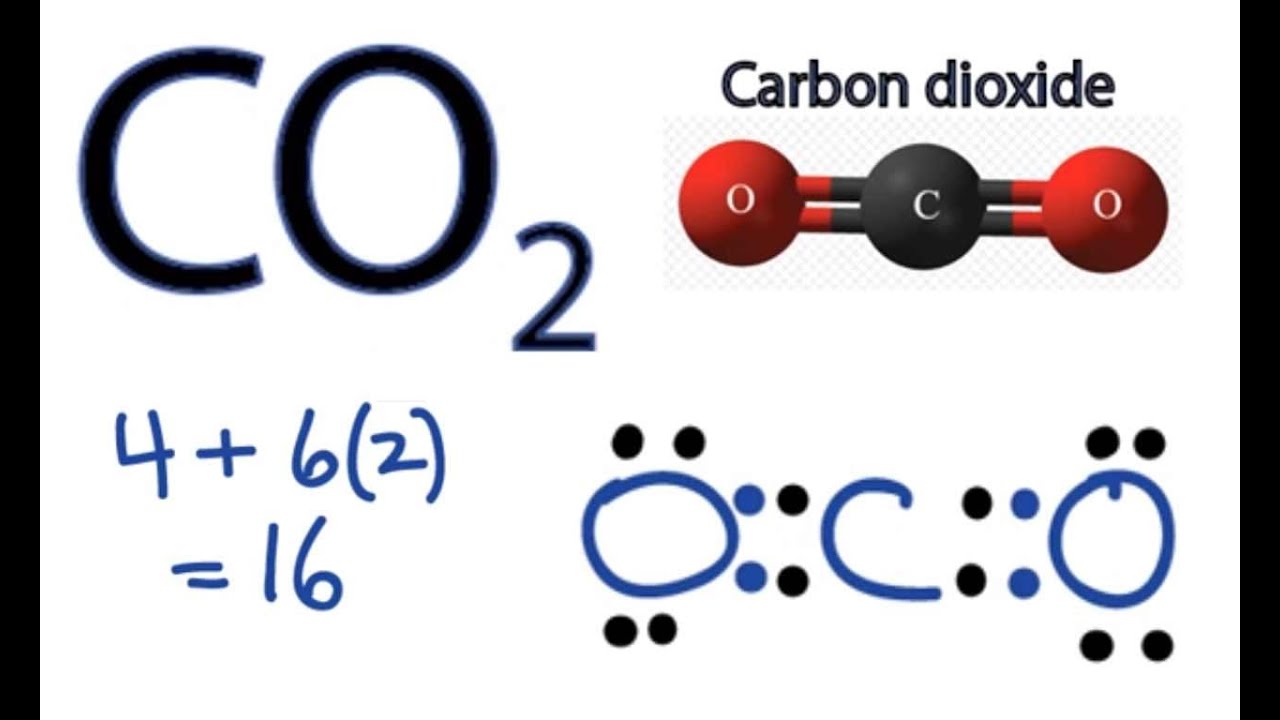
CO2 Lewis Structure How to Draw the Dot Structure for Carbon Dioxide
The CO 2 Lewis structure depicts the molecular arrangement of carbon dioxide, which is composed of one carbon atom and two oxygen atoms. Within the CO 2 Lewis structure, the carbon atom is surrounded by two double bonds, with each oxygen atom attached to it. Additionally, there are two lone pairs on each oxygen atom. To draw a CO 2 Lewis structure, begin by sketching out a rough structure of.

CO2 Lewis Structure ,Valence Electrons, Formal Charge ,Polar or
The Lewis dot structure of carbon dioxide is shown in Figure \(\PageIndex{8}\). Figure \(\PageIndex{8}\): Three resonance structures of carbon dioxide. In the above Figure we see the second and third resonance structures average out to the first, and so the average of all the resonance structures is a double bond. Thus it is common to write.

Carbon Lewis Dot Structure
Both carbon monoxide, CO, and carbon dioxide, CO 2, are products of the combustion of fossil fuels. Both of these gases also cause problems:. The Lewis structure of XeF 2 shows two bonding pairs and three lone pairs of electrons around the Xe atom: XeF 6: We place three lone pairs of electrons around each F atom, accounting for 36 electrons.

CO2 Lewis Structure ,Valence Electrons, Formal Charge ,Polar or
Lewis Structure of Carbon Dioxide. Carbon dioxide is a colourless, odourless, incombustible gas produced by the combustion of carbon. The carbon-oxygen ratio in a CO 2 molecule is 1:2. Two double bonds connect the carbon and oxygen atoms in the Lewis structure. Two oxygen atoms are present at the terminals, where they share electrons and form.

Carbon Dioxide Electron Dot Diagram General Wiring Diagram
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

So far, we’ve used 16 of the CO2 Lewis structure’s total 16 outermost
Step #1: Calculate the total number of valence electrons. Here, the given molecule is CO2 (carbon dioxide). In order to draw the lewis structure of CO2, first of all you have to find the total number of valence electrons present in the CO2 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).
Co Molecule Lewis Structure
This chemistry video explains how to draw the lewis structure of CO2 also known as Carbon Dioxide. It also discusses the bond angle, molecular geometry, and.
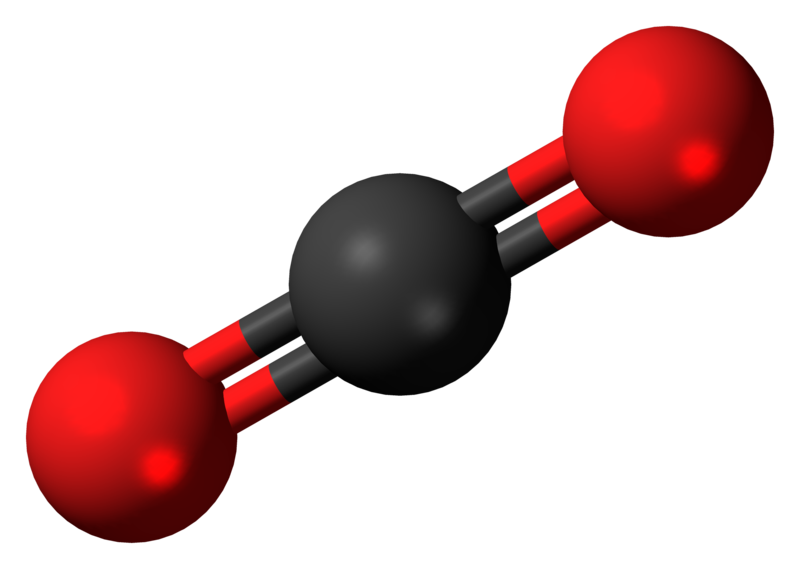
Carbon dioxide (CO2) Climate Encyclopedia
A step-by-step explanation of how to draw the CO2 Lewis Dot Structure (Carbon dioxide).For the CO2 structure use the periodic table to find the total number.

Draw The Lewis Structure Of Carbon Dioxide Co2 Fotodtp
I quickly take you through how to draw the Lewis Structure of CO2 (Carbon DiOxide). I also go over hybridization, shape and bond angles.